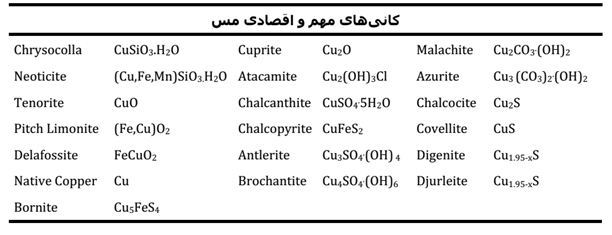
Copper is found in nature in two main forms, i.e. sulfide and oxide minerals and sometimes in free form. Almost all copper leachable deposits with sulfuric acid on the surface of the earth are formed due to weathering and oxidation of its primary sources. Most of the primary deposits of copper are formed in the deep parts of the earth’s crust under conditions of high temperature and pressure. In nature, the copper element combines with almost every element in the periodic table and forms minerals in the form of carbonates, silicates, hydroxides, oxides, chlorides, sulfates, phosphates, and sulfides. This characteristic of copper has caused various minerals and sources of this element to exist in nature. While the number of known minerals containing copper in nature is over a hundred, but the number of important and economic minerals is limited. Experience has proven that there are only two or three important minerals in a deposit with a significant amount of copper ore content. Important and economic minerals of copper are given in Table 1.
table 1- Important and economic minerals of copper
Oxide minerals or minerals that are formed in an oxidizing environment are diverse and can be subjected to a wide range of metallurgical operations. The most important copper oxide minerals are according to Table 2.
Table 2- Copper oxide minerals
1-2-1- لیچینگ اسیدی
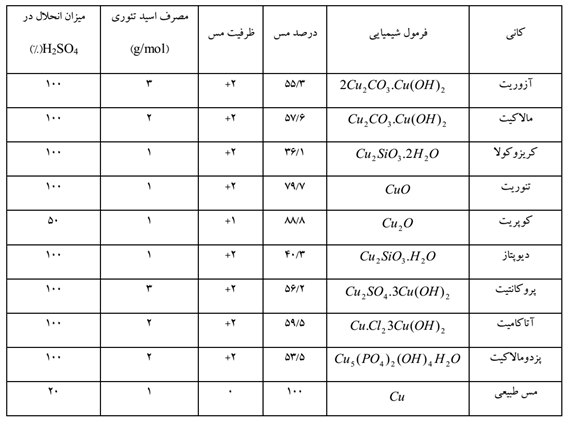
Dilute sulfuric acid is used on a large scale to dissolve copper oxide minerals. The leaching ability of some pure oxide minerals is given in Table 3.
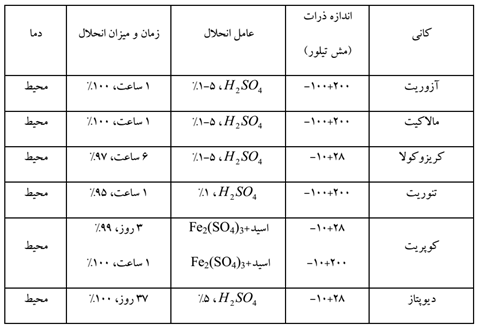
Azurite, malachite and tenorite dissolve quickly in dilute sulfuric acid solution. Chrysocolla dissolves more slowly in acid. Other copper silicate minerals like dioptase dissolve much slower, but the dissolution process can be accelerated with a stronger acid. Cuprite needs ferric sulfate for dissolution and only 50% of it dissolves in sulfuric acid. Natural copper is also dissolved with the help of ferric sulfate and leaching is possible only with sulfuric acid, only under aeration and high temperature.
Table 3- Ability to dissolve copper oxide minerals
– Azurite
(1) 2CuCO_3.Cu(OH)_2+3H_2 SO_4 →3CuSO_4+2CO_2+4H_2 O– Malachite
(2) CuCO_3.Cu(OH)_2+2H_2 SO_4 →2CuSO_4+CO_2+3H_2 O– Chrysocolla
(3) CuSiO_3.2H_2 O+H_2 SO_4 →CuSO_4+SiO_2+3H_2 O– Oven
(4) CuO+H_2 SO_4 →CuSO_4+H_2 O– Cuprite
(5) CuO+H_2 SO_4 →CuSO_4+Cu+H_2 O– brocanthite
(6) CuSO_4+3Cu(OH)_2+3H_2 SO_4 →4CuSO_4+CO_2+6H_2 ODuring the dissolution of cuprite mineral, natural copper is also formed, approximately 5% of which dissolves in acid. The remaining copper can be dissolved by acidic solution of ferric sulfate:
(7) Cu+〖Fe〗_2 (SO_4 )_3 →CuSO_4+2FeSO_4Most oxide minerals dissolve 2 to 3 times faster than secondary sulfide minerals in industrial operations. In the leaching process of copper oxide minerals, the rate of dissolution is controlled by the infiltration of H+ ions and copper ions out. The concentration of copper and acid have an opposite relationship from the surface of the particle to its interior. From the surface of the particle to the inside, the concentration of acid decreases and the concentration of copper increases.
2-2-1- Alkaline leaching
Copper oxide ores are faced with high consumption of acid and filtration problems due to the presence of carbonate tailings, and acid leaching is often problematic.
Advantages and disadvantages of the ammonia method
Ammonia washing has many advantages over acid washing, the most important of which are:
1- The obvious difference of the methods, i.e. performing operations in the play environment, makes it possible to use minerals with high carbonate which cannot be used in acid washing due to the high consumption of acid.
2- Problems related to corrosion
3- This type of process is suitable for mass washing of low-grade ore and tank washing of high-grade ore.
4- Since metals such as iron and manganese cannot be dissolved by ammonia or show a weak complexation ability with ammonia, ammonia leaching has a suitable selective power for the desired metal compared to the mentioned elements. If the high solubility of the mentioned elements in acidic agents leads to the high consumption of these agents and uneconomical washing process.
5- Ammonia also prevents the dissolution of calcium in the presence of carbonate and small amounts of sulfate.
6- Another element that causes problems in hip permeability and drainage is silica. Unlike acids, ammonia does not react with different compounds of this element such as alumina silicates and ferrosilicates.
7-After the termination of ammonia leaching, problems related to hip washing, neutralization and long term monitoring to prevent acid leakage are minimized. In addition, the remaining ammonia in the soil can act as a fertilizer for plant growth.
In addition to the above advantages, features such as low toxicity, low cost, easy recycling, and costeffectiveness are also considered to be the main factors in expanding the use of ammonia in hydrometallurgical processes, especially washing.
Disadvantages of ammonia laundry:
1- It is less washable than acidic compounds.
2- The use of ammonia is more difficult due to its high evaporation power compared to acidic compounds, although by using urea hydrolysis, the risk of transporting it can be reduced to a minimum.
